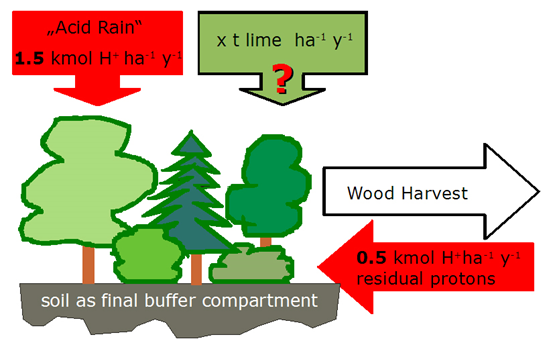
Problem: Acidification in Forest Ecosystems
In central and northern Europe the air cleaning effect of forests causes an increased input of acidity in forest ecosystems. The harvest of biomass also causes acidification because the harvested biomass and its „base potential” is not returned to the soil. This acidification has to be finally buffered by the forest. We have good reasons to suppose that the input of acidity (protons, H+) exceeds by far the buffer potentials of the soils. To prevent soil acidification, foresters try to compensate the input of acidity by liming the forest floor.
Quantifying Acidification and Buffering in Forest Ecosystems
We assume that the input of acidity corresponds to the rate exceeding the sustainable buffer potential of the soils and is
2 kmole H+ ha-1 year-1.
The soils are already so much acidified, that their sustainable ecological buffer potential is considered to be 0. Therefore, the input of acidity must be neutralized by lime if soil functions are to be preserved. The consumption of protons (H+ ) in the forest floor by lime can be given by the following simplified bulk reaction:
| CaCO3 | + 2 H+ | → Ca2+ | + H2O | + CO2 ↑ |
| lime | + protons | → Ca2+-ions | + water | + carbon dioxide |
This reaction contains two ecologically relevant information:
Qualitatively: Lime is dissolved by consumption of protons (H+) and the liberation of Ca²+-ions.
Quantitatively: Two proton atoms (2H+) or two Mol protons are neutralized by 1 molecule or one Mol lime (1 M CaCO3).*
* 1 mole lime (6.022 x 10²³ molecules CaCO3) weighs 40 + 12 + 3*16 = 100 g.